E-cigarettes: Facts, stats and regulations
E-CIGARETTES
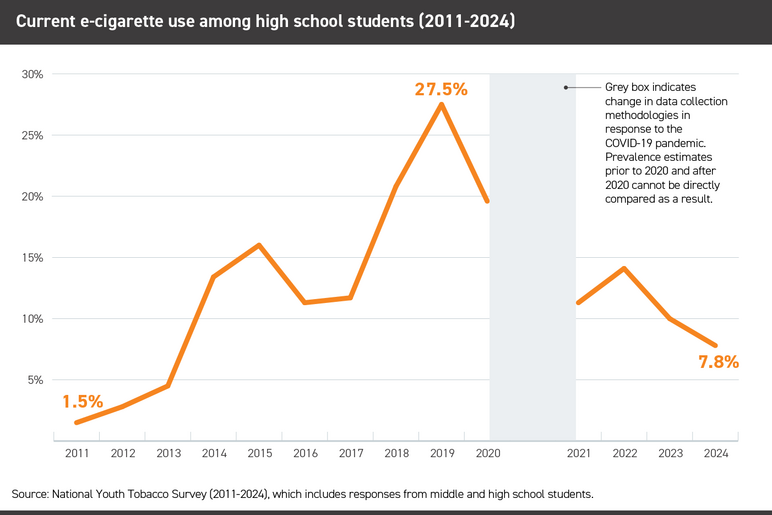
Although e-cigarettes have been sold in the U.S. for nearly 20 years, use patterns have shifted dramatically. As newer iterations brought higher levels of nicotine in an increasing array of flavors and product designs, youth use skyrocketed. In 2019, four years after JUUL introduced a new nicotine delivery system coupled with a relentless online, youth-focused marketing campaign, more than a quarter (27.5%) of high school students reported using e-cigarettes.
While e-cigarette use prevalence has declined significantly since the heyday of JUUL, an estimated 1.63 million middle and high school students currently use e-cigarettes, which remains a concern. Thousands of flavored, high-nicotine, and relatively cheap e-cigarette products remain on the market – many of them illegally – driving youth use and nicotine addiction. Equally concerning, among students who reported current e-cigarette use in 2024, 38.4% reported frequent use and 26.3% reported daily use.
Sustained e-cigarette use among young people can in part be credited to products remaining on the market illegally for years without U.S. Food & Drug Administration (FDA) authorization. While the FDA has denied permission to market some e-cigarette products and has recently stepped up enforcement efforts, including warning letters, fines, injunctions to manufacturers and retailers, and joint operations with other federal officials to seize imports of unauthorized e-cigarette products, enforcement has been slow and many of the most popular e-cigarettes among youth remain on the market.
E-cigarette use among adults has been more stable, with adult use remaining relatively low since around 2012. Recent data, however, showed a slight uptick in adult e-cigarette use from 4.5% in 2021 to 6% in 2022. While switching completely from cigarettes to e-cigarettes can be less harmful than smoking, many adult smokers use e-cigarettes and cigarettes concurrently — increasing overall exposure to nicotine. Randomized control trials provide growing evidence that e-cigarettes with nicotine can increase quitting rates when compared to nicotine replacement therapies. However, to date, no e-cigarette product has been approved by the FDA for quitting smoking.
This resource presents an overview of key aspects of e-cigarettes, including health effects, marketing practices, and regulations based on research and evidence available at the time of publication.

Thousands of flavored, high-nicotine, and relatively cheap e-cigarette products remain on the market —many of them illegally — driving youth use and nicotine addiction.
THE E-CIGARETTE MARKETPLACE
E-cigarettes are products that heat a solution that typically includes nicotine and flavoring. E-cigarettes first entered the market in 2007 and have evolved many times since then, with older versions designed to resemble regular cigarettes, cigars, pipes, and pens. E-cigarettes next evolved into “pod mods” with e-liquid sold in disposable pods. In 2015, the emergence of the brand JUUL took pod mods to the next level with high tech design, high-nicotine delivery, and youth- appealing marketing that hooked a new generation to nicotine.
Today's rapidly evolving e-cigarette market includes many varieties of disposable e-cigarettes, or single-use e-cigarettes, that do not use pre-filled pods containing e-liquid. The latest e-cigarettes also contain some of the highest nicotine levels ever seen in tobacco products. While some claim to be “tobacco-free” — made with lab-created synthetic nicotine, or with nicotine that does not come from tobacco — synthetic nicotine products still contain nicotine.
E-cigarette sales have rapidly escalated in the relatively short history of the products. Overall e-cigarette sales, as well as the total number of e-cigarette brands, increased by more than 46% between January 2020 and December 2022. Disposable e-cigarettes have nearly tripled in nicotine strength, quintupled in e-liquid capacity, and dropped in price by nearly 70% between 2017 and 2022. The number of unique e-cigarette products more than quadrupled from 453 in June 2021 to 2,023 in June 2022.
A Note on Language
E-cigarettes or electronic nicotine delivery systems (ENDS) go by many names — the most common name is “e-cigarette,” but other terms include e-cigs, vapes, vape pens, mods, and tanks. For the purposes of this resource, we refer to the entire category as “e-cigarettes.”
INDUSTRY MARKETING AND YOUTH TARGETING
Manufacturers and sellers of e-cigarettes aggressively target young people. There are few federal restrictions on e-cigarette marketing, allowing companies to promote their products through traditional outlets — such as TV and radio — despite a ban in 1971 on cigarette advertising in both outlets to reduce cigarette marketing to children. E-cigarette companies also take advantage of other marketing outlets, including the internet, retail environments, and recreational venues and events.
- In 2021, more than 75% of middle and high school students reported exposure to marketing or advertising of any nicotine or tobacco product — including e-cigarettes.
- Pro-e-cigarette content can increase perceptions that e-cigarettes are stylish, popular, safe for use, and have a pleasurable taste and smell.
- Exposure to such content can normalize and glamorize e-cigarette use and has been strongly associated with current e-cigarette use among young adults.
- E-cigarette companies have appealed to youth by marketing kid-friendly candy and food- flavored products resembling toys, food, or cartoon characters.
- E-cigarettes are promoted heavily through retail stores, online (e.g., YouTube, Twitter and mobile ads), and through TV ads.
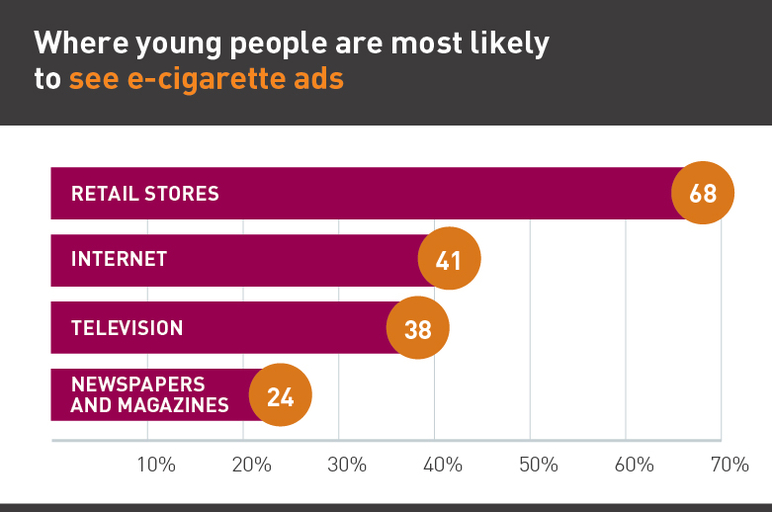
How e-cigarette content and marketing is infiltrating social media
Social media platforms enable branded or promotional content from commercial brands, peers, or influential networks to spread virally, which can increase youth exposure to pro-tobacco content and advertising. Social media marketing of e-cigarettes began in earnest when the makers of the popular e-cigarette JUUL conducted a massive influencer engagement campaign as part of the product’s 2015 launch, employing an entire department dedicated to social media influencer marketing. Efforts to inundate young people with e-cigarette content on social media continue today:
- Influencers on Instagram frequently posted branded vaping content that promoted specific brands, failed to disclose brand relationships, rarely included warning labels about the addictiveness of nicotine, and featured youth-appealing cartoon imagery, violating both federal and platform regulations.
- Vaping advocates played a key role in amplifying and re-tweeting misleading content about tobacco, nicotine, and COVID-19 on Twitter.
- Vaping advocates on Twitter used tweets by the FDA about e-cigarette regulation to spread anti-regulatory messages. Rebuttal messages by vape advocates were disseminated far more broadly than the FDA’s original tweets.
For more information on how tobacco content is infiltrating social media, see “Industry influencer: How tobacco content is infiltrating social media.”
WHO IS USING E-CIGARETTES?
YOUTH
E-cigarettes have been the most commonly used tobacco product among U.S. youth since 2014. E-cigarette use among middle and high school students steadily increased from 2011, reaching its zenith at 27.5% of high school students using e-cigarettes in 2019. Current e-cigarette use among middle and high school students declined significantly between 2023 and 2024 (7.7% to 5.9%, or 2.13 million to 1.63 million) but e-cigarette use remains a serious public health threat: among students who currently used e-cigarettes in 2024, 38.4% reported frequent use and 26.3% reported daily use.
- In 2024, 3.5% of middle school students and 7.8% of high school students – roughly 1.63 million students – reported current (i.e., past 30-day) e-cigarette use.
- Among current e-cigarette users:
- 38.4% reported frequent use, and 26.3% reported daily use.
- 87.6% used a flavored e-cigarette
- Disposable e-cigarettes (55.6%), prefilled or refillable pods or cartridges (15.6%), and tanks or mod systems (7.0%) were the most commonly used device types. 21.8% of students were unsure of the device type used.
- In 2022, young people most commonly acquired e-cigarettes through social sources (56.9%) followed by retail sources (43.1%).
While e-cigarette use among young people has declined in recent years, it remains a serious public health threat: 10% of high school students used e-cigarettes in 2023, many of whom were not smokers in the first place.
ADULTS
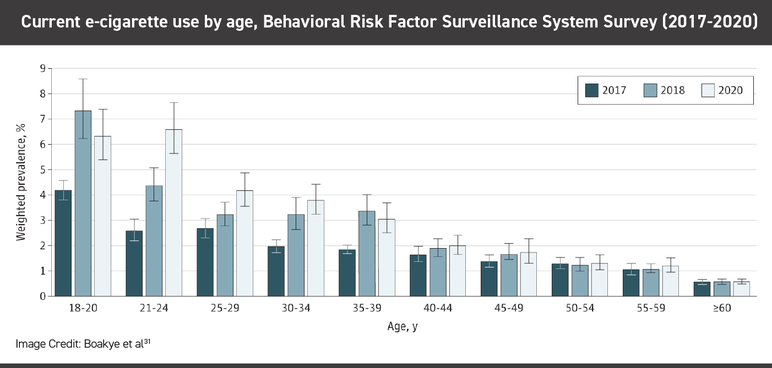
E-cigarette use has remained relatively low and stable among adult users since around 2012, although use has gone up slightly among adults recently, from 4.5% in 2021 to 6% in 2022. In 2022, the greatest e-cigarette use among adults was among 23- to 24-year-olds, 22.8% of whom were current users, according to Monitoring the Future.
HEALTH EFFECTS OF E-CIGARETTE USE
While some e-cigarette brands have recently begun to offer nicotine-free products, most e-cigarettes products sold in the U.S. contain nicotine. Nicotine is harmful to developing brains and its use during adolescence can disrupt the formation of brain circuits that control attention, learning, and susceptibility to addiction. Exposure to nicotine among youth is particularly dangerous since it affects key brain receptors, making young people more susceptible to nicotine addiction.[For more information on nicotine, see “ Nicotine and the Young Brain.”]
When evaluating the risks of e-cigarette use, it is important to note that exposure to harmful chemicals inhaled by e-cigarette users can vary greatly depending on e-cigarette device characteristics (voltage, flavor, nicotine) and how the device is used. Here is what we know about the potential health risks associated with e-cigarette use, based on recent findings from a 2018 National Academies of Sciences, Engineering, and Medicine report as well as other major reviews on the topic. However, because studies included in reviews can vary drastically, it is important to note that additional research is needed on the health effects of e-cigarettes.
- Asthma
There is moderate evidence for increased coughing and wheezing in adolescents who use e-cigarettes. E-cigarette use is associated with an increase in asthma exacerbations. Two meta-analyses have identified an association between current and ever e-cigarette use and asthma. However, significant inconsistencies across studies suggest the need for additional research. - Cancer
Some chemicals present in e-cigarette aerosols, such as formaldehyde and acrolein, can cause DNA damage and mutagenesis that can lead to cancer. Long-term exposure to e-cigarette aerosols could increase the risk of cancer and adverse reproductive outcomes; however, given that studies varied, additional research is needed. - Heart Disease
A meta-analysis found an association between e-cigarette use and heart attack, although the risk of e-cigarettes causing a heart attack was lower than the risk of heart attack from cigarette smoking. A 2023 meta-analysis concluded that inhalation of e-cigarette aerosol leads to impaired functioning of the lining of blood vessels. To date, there is no available evidence to suggest that e-cigarette use is associated with clinical cardiovascular outcomes such as coronary heart disease, stroke, peripheral artery disease, and early stages of atherosclerosis. -
Stroke, Lung Function and Other Conditions The role of e-cigarette use in the development of stroke remains inconclusive. Short-term e-cigarette inhalation has a similar effect on lung function as cigarettes and vaping may increase airway resistance in lungs. However, given the limited size and follow-up duration of existing studies, more research is needed.
Youth e-cigarette use is also linked to future cigarette use. Young people in the U.S. who had ever used e-cigarettes in 2018 had 7x higher odds of ever using cigarettes and 8x higher odds of currently using cigarettes one year later, compared with those who had never used an e-cigarette, according to Truth Initiative research.
E-CIGARETTE REGULATION
FEDERAL
E-cigarettes have been allowed to stay on the market for years without undergoing a full review of their public health impact, sparking a sustained and ongoing crisis of youth use. As of November 2023, the FDA has not completed reviewing all applications from e-cigarette makers to market their products, leaving many e-cigarettes on the market while waiting for a decision. To date, the FDA has denied permission to market flavored and menthol e-cigarette products such as Vuse Solo, Vuse Vibe, Vuse Ciro, and Vuse Alto. The FDA has denied permission to market many non-tobacco and non-menthol flavored e-cigarette products, however, many of those denials are currently under judicial or supervisory review. In recent years, the FDA has increased enforcement efforts around e-cigarette manufacturers and retailers for continuing to sell youth-appealing e-cigarettes. These actions include issuing warning letters, fines, injunctions, and joint operations with other federal officials to seize imports of unauthorized e-cigarette products.
See “ Dangerous Delays: The Failure to Regulate E-Cigarettes” for a complete timeline.
STATE AND LOCAL REGULATION
State and local authorities can restrict the sale of flavored tobacco products, including e-cigarettes. Such policies are critical in the absence of strong federal regulation of e-cigarettes. As of March 31, 2023, seven states and an estimated 388 counties, cities, towns, and tribes have restricted the sale of flavored tobacco products. However, this leaves a large majority of the U.S. population not covered by such restrictions.
See “ Local restrictions on flavored tobacco and e-cigarette products ” for a full report on local flavored tobacco policies.
TAXES
Although there is no federal excise tax on e-cigarettes, states have the authority to tax e-cigarettes. Thirty-two states, the District of Columbia, and two territories have imposed a tax on e-cigarettes as of June 15, 2023.
SMOKE-FREE AIR LAWS
Seventeen states, D.C. and Puerto Rico have passed comprehensive smoke-free indoor air laws that include e-cigarettes, as of March 31, 2023. These laws prohibit smoking and the use of e-cigarettes in indoor areas of private worksites, restaurants, and bars.

Big Tobacco’s So-Called “Harm Reduction” Strategy
The tobacco industry has co-opted the term harm reduction to further its own interests of reduced tobacco product regulation and expand its user base. Tobacco companies have seized the concept of harm reduction — the proven public health approach of providing evidence-based lower harm alternatives for those who don't quit harmful substances — as a proxy for a vision of a lightly regulated market of nicotine products that provides for continued and robust growth of nicotine as a commercial product.
Truth Initiative supports regulation that encourages the development of consistently safer nicotine delivery alternatives that allow smokers to quit tobacco altogether or switch completely to a much less harmful, well-regulated product. Truth Initiative forcefully rejects, however, the notion that this requires the further development of a huge commercial market in addictive nicotine products focused on growth and the acquisition of new users, most of whom are youth and young adults. Because the youth e-cigarette crisis in the United States and the youth appeal of flavored e-cigarettes go hand in hand, Truth Initiative strongly supports removing all flavored e-cigarettes from the market, regardless of device type.
IMPACT ON SMOKING CESSATION
To date, no e-cigarette product has been approved by the FDA for smoking cessation. In contrast, nicotine replacement therapies (e.g., patch, gum, lozenge) and some prescription medications (i.e., varenicline, bupropion) have been evaluated by the FDA and found to be safe and effective at helping people quit smoking.
There is limited evidence to date that e-cigarettes may be effective aids to support smoking cessation. Some studies have shown that e-cigarettes may help smokers quit using cigarettes, while others have found e-cigarettes to be ineffective. A 2022 meta-analysis of 61 studies reported with high certainty that people using nicotine e-cigarettes had higher quit rates, compared to people randomized to using nicotine replacement therapy. Another 2021 meta-analysis found that randomized controlled trials offering free e-cigarettes to research participants increased likelihood of cessation. The same meta-analysis noted that e-cigarette use was not associated with increased smoking cessation among observational study designs. These conflicting findings may be explained by differences in participant selection and environmental context across studies.
See “ Quitting Tobacco ” for more information on quitting tobacco products.

STRATEGIES TO REDUCE YOUTH E-CIGARETTE USE
MASS MEDIA CAMPAIGNS
Mass media campaigns such as truth ® have been highly effective in helping to reduce youth smoking. truth has prevented millions of young people from becoming smokers, including 2.5 million between 2015 and 2018 alone. A growing body of research indicates that truth campaigns to prevent young people from vaping are poised to move in the same direction as the organization’s successful smoking prevention campaigns.
- A truth campaign focused on the connection between vaping nicotine and mental health significantly reduced e-cigarette use and also lowered odds of current e-cigarette use among young people.
- Young people with strong truth brand awareness and loyalty had significantly lower odds of vaping and intending to vape a year and half later.
- truth vaping prevention campaigns have successfully increased e-cigarette knowledge and shifted beliefs. Young people who had seen truth campaigns were more likely to believe that e-cigarettes were harmful and socially unacceptable and hold anti-tobacco industry attitudes compared to those unaware of the campaign.
VAPING CURRICULUM
An online course about the dangers of using e-cigarettes, created by Truth Initiative and Kaiser Permanente and in collaboration with the American Heart Association, significantly increased e-cigarette knowledge among middle and high school participants. This free digital learning experience is part of Truth Initiative’s nationally recognized truth campaign and is made available to schools by leading social impact education innovator, EVERFI. A Truth Initiative study finds that students who completed the digital course Vaping: Know the truth saw a 15% average increase in their scores on e-cigarette knowledge compared to before completing the course.
QUIT VAPING SUPPORT
Youth and young adult e-cigarette users, many of whom never previously used tobacco, also need support to quit. Studies have found that nearly two-thirds of adult e-cigarette users plan to quit, and almost a quarter of adolescents want to quit and have made a quit attempt.
This is Quitting, now part of EX Program, is a free and anonymous text messaging program from Truth Initiative designed to help young people quit vaping. The first-of-its-kind quit program has helped over 780,000 youth and young adults quit vaping by incorporating messages from other young people like them who have attempted to, or successfully quit, e-cigarettes.
Research shows that This is Quitting works: The quit-vaping program increased odds of quitting among young adult e-cigarette users aged 18-24 by nearly 40% compared to a control group, according to a 2021 randomized clinical trial published in JAMA Internal Medicine in 2021. A separate study published in 2024 found that participants who used the program were 35% more likely to report not using nicotine after the 7-month study period, compared to a control group.
The quit vaping program also ensures that young people don’t later use combustible tobacco products in place of e-cigarettes. More young e-cigarette users who used This is Quitting abstained from using e-cigarettes and combustible tobacco products (25.9%) compared to a control group that did not receive quitting support (18.5%), according to research published in Preventive Medicine.
This is Quitting is tailored based on age (13-24 years old) and product usage to give teens and young adults appropriate recommendations about quitting. Teens and young adults can join for free by texting DITCHVAPE to 88709.
In addition to vaping prevention campaigns and quitting support, much work remains for federal, state, and local governments to establish comprehensive policies to help protect youth from a lifetime of nicotine addiction, including removing all non-tobacco flavors from the market, ensuring a thorough and transparent FDA review of all products, and placing restrictions on e-cigarette marketing.
See “Action needed on e-cigarettes” for more information.

Studies have found that nearly two-thirds of adult e-cigarette users plan to quit, and almost a quarter of adolescents want to quit and have made a quit attempt.

More in emerging tobacco products
Want support quitting? Join EX Program
By clicking JOIN, you agree to the Terms, Text Message Terms and Privacy Policy.
Msg&Data rates may apply; msgs are automated.


